Refrigerants
1) What is refrigerant and why is it used?
Refrigerant is a fluid used for heat transfer in a refrigerating system that absorbs heat during evaporation from a region of low temperature and low pressure and releases heat during condensation at a region of high temperature and high pressure.
2) Classification of Refrigerant and explain each type.
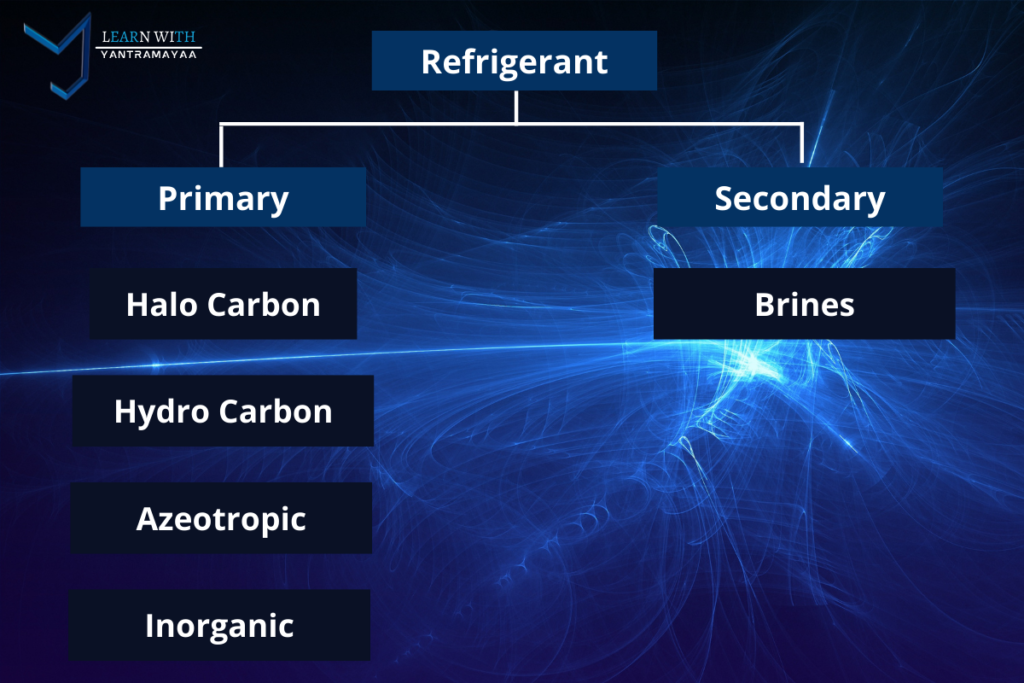
Primary Refrigerant: Primary refrigerants are the refrigerants which cools the substance or space by directly absorbing latent heat.
It absorbs heat from evaporator during evaporation and releases heat to condenser during condensation.
examples: Ammonia, Co2
Halo Carbon:
- They are fluorocarbons of methane and ethane series.
- Contains 1 or more of theses halogens (fluorine, chlorine, bromine)
- Excellent thermodynamic properties
- Damages ozone layer
examples: R11, R12, R22, R123
Hydro Carbon:
- These are natural and non toxic
- No ozone depletion properties
- Save cost
- More efficient conductors of heat than fluorocarbon refrigerants
examples: R50, R600, R290
Azeotropic:
- This group of refrigerant consists of mixture of different refrigerants which cannot be separated under pressure and temperature and have fixed thermodynamics properties
- Designated by 500 series
examples: R-500, R-502, R-503
Inorganic:
- In use before the introduction of halo carbons and are still used.
examples: Ammonia, air, H2o, Co2
Secondary Refrigerant: Used as a cooling medium which absorbs heat from refrigerated space and transfer to the primary refrigerant in evaporator.
Also known as Brines or Antifreezes.
examples: Ammonia, air, H2o
3) State properties of refrigerants
Thermodynamic Properties:
- Critical Temperature and Pressure
- Boiling Temperature
- Freezing Temperature
- Evaporator and Condenser pressure
- Latent heat of vaporization
- Specific Volume
Chemical Properties:
- Flammability
- Toxicity
- Miscibility with oil
- Solubility of water
- Effect on stored product
Physical Properties:
- Stability
- Viscosity
- Thermal conductivity
- Corrosiveness
- Dielectric strength
- Leakage tendency
- Performance
- Cost
4) State desirable characteristics of refrigerants
Desirable Properties of Refrigerants:
- Low boiling point
- High Critical temperature
- High latent heat of vaporization
- Low specific heat o liquid
- Low specific volume of vapor
- Non corrosive to metal
- Non toxic
- Non flammable and non explosive
- Less costly
- Easy to liquify at moderate pressure and temperature
- Easy to locate leaks by odour or suitable indicator
- Mixes well with oil
- Low solubility of water
- Good dielectric strength
5) Explain GWP and ODP of refrigerants
GWP (Global Warming Potential): It is the measure of relative global warming effects of different gases.
It assigns a value to the amount o heat trapped by a certain mass of a gas relative to the amount of heat trapped by a similar mass of carbon dioxide over a specific period of time.
The higher the GWP value, the more that particular gas warms the Earth compared to carbon dioxide.
ODP (Ozone Depletion Potential): It is the ratio of impact on ozone layer of a chemical compared with the impact of the similar mass of R11.



No responses yet